Organic Chemistry Online
A
Acid:
any species capable of producing a positively charged hydrogen
ion (H+). In an aqueous medium the H+ usually exists in a solvated form
(H3O+) called the hydronium or hydroxonium ion.
Achiral: not chiral.
A compound (or object) that is superimposible on its mirror image. Example:
Activation energy:
the miminmum energy which reacting species must possess in order
to be able to form an 'activated complex', or 'transition state', before
proceeding to the products. Activation energy is influenced by reaction
conditions such as temperature, pressure, catalysts etc.
Addition reaction:
a reaction in which an unsaturated system is saturated or part saturated
by the addition of a molecule across the multiple bond.[E.g. the addition
of bromine to ethene to form 1,2-dibromoethane]
Alkaloid: One of
a varied family of alkaline, nitrogen-containing substances, usually plant-derived,
reacting with acids to form salts. Normally intensely
bitter, alkaloids form a body of substances widely used in drug and herbal
therapy. Examples include caffeine, morphine, codein etc. .
Allyl group: a group
containing 3 carbon atoms and a double bond.
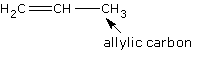
Allylic rearrangement: the
migration of a double bond in a 3-carbon system from carbon atoms 1 and
2 to carbon atoms 2 and 3.
![]()
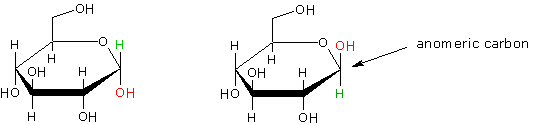
Anomers: the specific term used to describe carbohydrate stereoisomers differing only in configuration at the hemi-acetal carbon atom. Example is the anomeric forms of D-glucose.
Aromaticity: the
special property of planar (or nearly planar) cyclic, conjugated systems
having (4n+2) conjugated pi electrons. For benzene, the most common aromatic
system (n = 1, therefore 6 pi electrons. The delocalised pi electrons
gives aromatic compounds a special stability, hence they tend to undergo
substitution rather than addition reactions.
Association: a term applied to the combination of molecules of a substance with one another to form more complex systems.
Asymmetric:
a term applied an object or molecule that does not possess symmetry.
A symmetric induction:
a term applied to the selective synthesis of one diastereomeric form of
a compound resulting from the influence of an existing chiral centre adjacent
to the developing asymmetric carbon atom. This usually arises because,
for steric reasons, the incoming atom or group does not have equal access
to both sides of the molecule. See nucleophilic
substitution reactions.
Atomic orbital: the
energy levels of electrons in an atom which may be described in terms
of the four quantum numbers.
Avogadro's constant:
the total number of particles (atoms or molecules) in one mole of any
pure substance i.e.
![]()
B
Base: an organic
base is a species that can produce a hydroxide ion (OH-) or combine with
a proton to from a salt.
Benzene: a colourless, rather
pleasant-smelling, volatile and flammable liquid. It has the chemical
formula C6H6 and is used primarily
in the production of plastics and other chemical products. Benzene is
also a known human carcinogen, causing various types of leukemia, lymphoma,
and blood diseases.
Bimolecular reaction:
a chemical reaction in which two species (e.g. molecules, ions or radicals)
react to form new species. Most reactions are bimolecular or proceed through
a series of bimolecular steps.
Bond energy: the
energy required to break a particular bond by a homolytic process.
Buffer solution:
an equilibrium solution made of a weak acid or base and its conjugate
salt, and its pH only alters slightly when small quantities of acids or
bases are added.
C
Canonical structures:
any of two or more hypothetical structures of resonance theory
which can be written for a molecule simply by rearranging the valence
electrons of the molecule. [E.g. the two Kekule structures of benzene.
They are sometimes called 'valence bond isomers']
Catalyst: a substance
that, when added to a reaction mixture, changes (speeds up) the rate of
attainment of equilibrum in the system without itself undergoing a permanent
chemical change.
Catalytic cracking:
the method for producing gasoline from heavy petroleum distillates. [Generally
the catalysts are mixtures of silica and alumina or synthetic conjugates
such as the zeolites.]
Catalytic reforming:
the process of improving the octane number of straight-run gasoline by
increasing the proportion of aromatic and branched chain alkanes. [Catalysts
employed are either molybdenum-aluminium oxides or platinum based.]
Chain reaction: reactions
which proceed by means of a set of repeating cyclical steps, e.g. the
free radical addition of hydrogen bromide to an alkene.
Chirality: a term
which may be applied to any asymmetric object or molecule. The property
of non-identity of an object with its mirror image.
Chromatography: a
series of related techniques for the separation of a mixture of compounds
by their distribution between two phases. In gas-liquid chromatography
the distribution is between a gaseous and a liquid phase. In column chromatography
the distribution is between a liquid and a solid phase.
Compound:
a term used generally to indicate a definite combination of elements
into a more complex structure (a molecule) but it is also applied to systems
with non-stoichiometric proportions of elements.
Configuration: the
order and relative spatial arrangement of the atoms in a molecule. Absolute
configuration is when the relative 3 dimensional arrangement in space
of atoms in a chiral molecule have been correlated with an absolute standard.
Configurational isomers:
a series of compounds which have the same constitution and bonding
of atoms but which differ in their atomic spatial arrangement. [E.g. glucose
and mannose are configurational isomers. Also called stereoisomers. See
also positional or structural isomers.]
Conformation: the
spatial arrangement of a molecule in space at any particular moment in
time. [Most molecules can adopt an infinite number of conformations because
of the possible rotation about single covalent bonds. Of these possibilities
most compounds tend to spend most time in only one or a few conformational
states, called the preferred conformations.]
Conformer: a conformation
of a molecule; generally these will be at energy minima.
Conjugation: a sequence
of alternating double (or triple) and single bonds. (E.g. C=C-C=C and
C=C-C=O). Conjugation can also be relayed by the participation of lone
pairs of electrons or vacant orbitals.
Co-ordinate bond:
the linkage of two atoms by a pair of electrons both electrons being provided
by one of the atoms (the donor). [Coordinate bonds are covalent bonds.]
Constitution: the
number and type of atoms in a molecule.
Covalent bond: the
linkage of two atoms by the sharing of two electrons.
D
Delocalization: electron
systems in which bonding electrons are not localised between two atoms
as for a single bond but are spread (delocalized) over the whole group.
[E.g pi-bond electrons, in particular the delocalised pi-electrons associated
with aromatic molecules.]
Dextrorotatory: the
phenomenon in which plane polarised light is turned in a clockwise direction.
diastereomers (or diastereoisomers): stereoisomeric structures which are
not enantiomers (mirror images) of one another. [Often applied to systems
which differ only in the configuration at one carbon atom, e.g. meso-
and d- or l-tartaric acids are diastereoisomeric.]
Dihedral angle: the
angle between groups attached on adjacent carbon atoms when viewed in
a Newman projection.
Disproportionation:
a process in which a compound of one oxidation state changes to compounds
two or more oxidation states [E.g. 2Cu+ --> Cu + Cu2+]
Dissociation: the
process whereby a molecule is split into simpler fragments which may be
smaller molecules, atoms, free radicals or ions.
Dissociation constant:
the measure of the extent of dissociation, measured by the dissociation
constant K. For the process:
AB = A + B. K = ([A][B])/[AB]
Dissymmetric: chiral
Double bond: some
atoms can share two pairs of electrons to form a double bond (two covalent
bonds). Formally the second (double) bond arises from the overlap of p
orbitals from two atoms, already united by a sigma bond, to form a pi
bond.
Dyestuffs: intensely
coloured compounds applied to a substrate. Colours are due to the absorption
of light to give electronic transitions.
E
Eclipsed:
a conformation in which substituents on two attached saturated carbon
atoms overlap when viewed as a Newman projection.
Electronegativity: the tendency
for atoms in a molecule to attract electrons. [Measured in terms of the
HOMO and LUMO energy levels.]
Electronic configuration:
the particular order in which electrons are arranged in an atom or molecule.
[Used in a distinct and different sense from stereochemical configuration.]
Electronic transition: in
an atom or molecule the electrons have certain allowed energies only (orbitals).
If an electron passes from one orbital to another an electronic transition
occurs and there is the emission or absorption of energy corresponding
to the difference in energy of the two orbitals.
Electrophile: an atom, molecule
or ion able to accept an electron pair.
Electrophilic substitution:
an overall reaction in which an electrophile binds to a substrate with
the expulsion of another electrophile. [The most common example is the
electrophilic substitution of a proton by another electrophile, such as
a nitronium ion, on an aromatic substrate such as benzene.
Electrovalent (ionic) bond:
bonding by electrostatic attraction.
Element: a substance which
cannot be further subdivided by chemical methods.
Enantiomers: a pair of isomers
which are related as mirror images of one another. [E.g. isomers differing
only in the configuration about the chiral atoms.]
Endothermic: a reaction
in which heat is absorbed.
Energy diagram (or reaction energy diagram):
a graph of the energy of a reaction against the progress of the reaction.
Enthalpy: a thermodynamic
state function, generally measured in kilojoules per mole. In chemical
reactions the enthalpy change (deltaH) is related to changes in the free
energy (deltaG) and entropy (deltaS) by the equation:
deltaG = deltaH - T.deltaS
Entropy: a thermodynamic
quantity which is a measure of the degree of disorder within any system.
[The greater the degree of order the higher the entropy; for an increase
in entropy S is positive. Entropy has the units of joules per degree K
per mole.]
Enzyme: a naturally occurring
substance able to catalyse a chemical reaction.
Epimerization: a process
in which the configuration about one chiral centre of a compound, containing
more than one chiral atom, is inverted to give the opposite configuration.
[The term epimers is often used to describe two related compounds which
differ only in the configuration about one chiral atom.]
Epoxidation: the addition
of an oxygen bridge across a double bond to give an oxirane. [Achieved
by use of a peracid or, in a few cases, by use of a catalyst and oxygen.]
Equilibrium constant: according
to the law of mass action, for any reversible chemical reaction:
aA + bB = cC + dD, the equilibrium constant (K)is defined as:
K = ([C]c[D]d)/([A]a[B]b)
Excited state: the state
of an atom, molecule or group when it has absorbed energy and become excited
to a higher energy state as compared to the normal ground state. The excited
state may be electronic, vibrational, rotational, etc.
F
Fischer projection:
a convention for drawing carbon chains so that the relative 3-dimensional
stereochemistry of the carbon atoms is relatively easy portrayed on a
2-dimensional draw
Free energy (deltaG): a
thermodynamic state function; the free energy change (deltaG) in any reaction
is related to the enthalpy and entropy:
deltaG = deltaH - T.deltaS
Free radicals: molecules
or ions with unpaired electrons and hence generally extremely reactive.
['Stable' free radicals include molecular oxygen, NO, and NO2. Organic
free radicals range from those of transient existence only to very long-lived
species. Alkyl free radicals tend to be very reactive and short lived.]
Frontier orbital symmetry:
the theory that the site and rates of reaction depend on the geometries,
sign of the wave function and relative energies of the highest occupied
molecular orbital (HOMO) of one molecule and the lowest unoccupied molecular
orbital (LUMO) of the other.
Back to Top
Features | Glossary | Quiz