Organic Chemistry Online
Introduction
Nomenclature of Alcohols
Classification of Alchols
Physical Properties of Alcohols
Synthesis of Alcohols
Reactions of Alcohols
Uses of Alcohols
Introduction
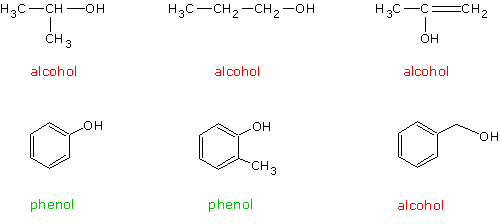
The functional
group in alcohols is the hydroxyl (OH) group. In alcohols the OH group
is attached to a carbon in a straight chain or cyclic hydrocarbon.
Compounds in which the OH is directly attached to a carbon that is part
of a benzene
ring are called phenols.
Nomenclature of Alcohols
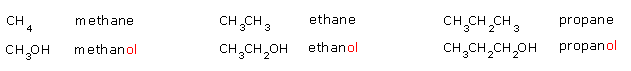
Alcohols are named by first naming the hydrocarbon chain
and replacing the final -e with the suffix -
ol. e.g.
More complex alcohols are named in accordance
with the IUPAC
set of rules which are summarized as follows:
1. Identify the longest continuous chain
of carbons to which the OH group(s) is/are attached to one of the carbons
in the chain.
2. Number the chain so that the hydroxyl group(s) is/are attached
to the lowest numbered carbons.
3. Identify and locate any other branches on the chain so that they
are named alphabetically and their carbon number is hyphenated onto the
front of the name. If more than one of the same group is present use the
greek prefix attached to the branch name. (di=2, tri=3, etc)
4. After all the branches have been named and located then attach
the carbon number that is attached to the hydroxyl group onto the alkane
name associated with the number of carbons found in the continuous chain
in step 1
5. For polyhydric alcohols you would have to locate two or more
carbons which the Hydroxyl groups were attached and hyphenate those carbon
numbers to the front of the alkane name and attach "diol" if
two are involved, "triol" if three hydroxyl groups were involved,
etc.
Examples:

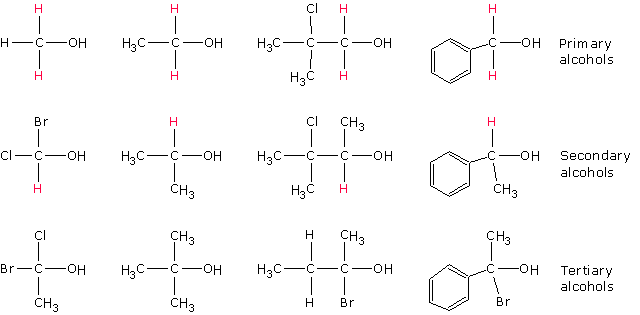
Alcohols are classified as primary, secondary or tertiary based on the type of carbon atom to which the OH group is attached. If the carbon bearing the OH group is attached to at least two hydrogen atoms, then the alchol is classified as primary. For secondary alcohols, the carbon bearing the OH group is attached to only one hydrogen atom whilst for tertiary alcohols, the OH bearing carbon has no hydrogen atom attached to it.
Physical Properties of Alcohols
The polar nature of the O-H bond (due
to the electonegativity difference of the atoms ) results in the formation
of hydrogen bonds with
other alcohol molecules or other H-bonding systems (e.g. water). The implications
of this are:
- high melting and boiling points compared to analogous
alkanes and alkyl halides
- high solubility in aqueous media
The table below compares the boiling points (°C) of alcohols with corresponding alkanes and alkyl halides.
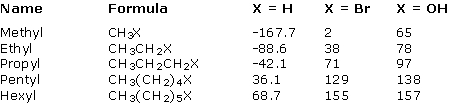
Generally, as the carbon chain increases in length, the
boiling points also increase as a result of increases in van der waals
forces. Branching tends to lower boiling points as van der waals forces
are lowered as a result of the molecules becoming more spherical. Polyhydric
alcohols have higher melting and boiling points than the monohydric counterparts.
Solubility of alcohols in water on the other hand tends to decrease as the carbon chain increases. The table below gives the solubilities of some alcohols in water (mol/100g of water).
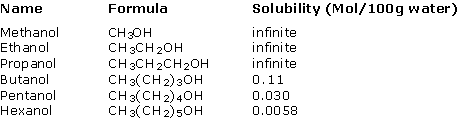
Notice that the solubility for the first three compounds, methanol, ethanol and propanol, are listed as infinite. These three alcohols are completely misible with water in all proportions. Up to about three carbon atoms, the solubility properties of these compounds are dominated by the OH group. Beyond three carbons, the organic, hydrophobic, portion of the molecule begins to affect the solubility and the OH group lessens in importance. By the time we get up to seven carbons, heptanol, the solubility of the compound in water is almost zero.
Synthesis of Alcohols
Alcohols can be synthesized in the laboratory through the use of various reagents and methods.
1. Hydration of Alkenes
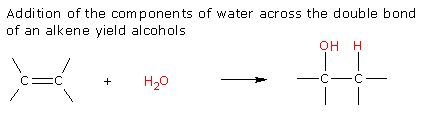
Several methods can be used to achieve this.
a). Acid catalyzed hydration
b). Oxymercuration-Demercuration
c). Hydroboration Oxidation
2. Hydroxylation of Alkenes
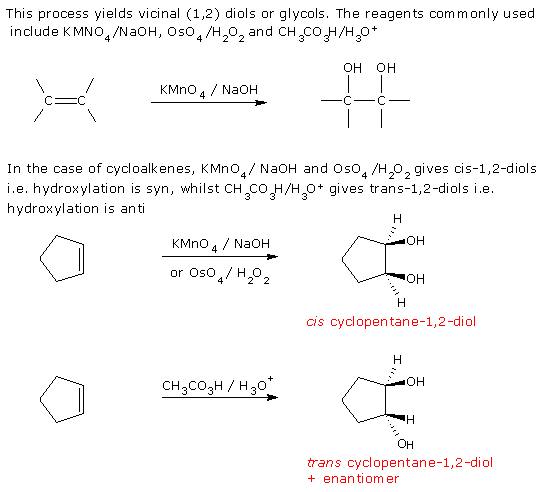
3. Reduction of Carbonyl
Compounds
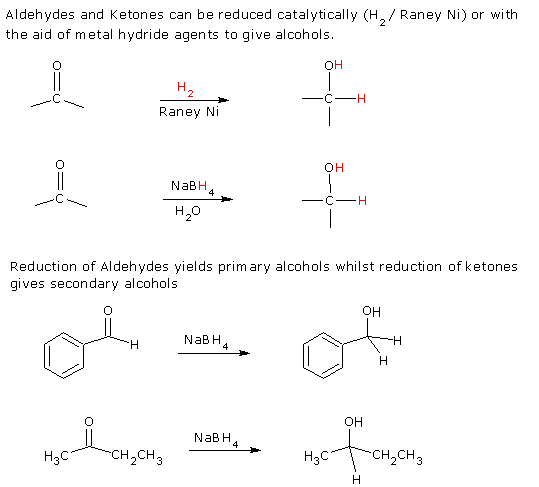
Reactions of Alcohols
1. Nucleophilic substitution reactions (movie). Download shockwave if movie does not appear
2. Dehydration.
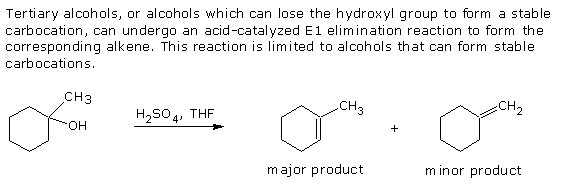
3. Oxidation.
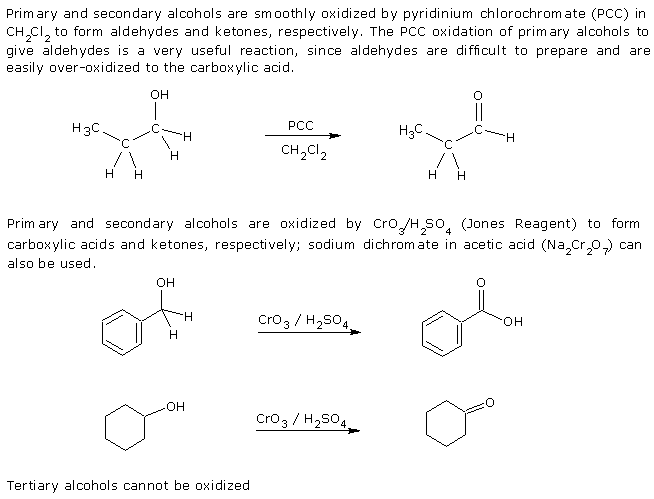
Uses of Alcohols
The main uses of alcohols are as solvents for gums, resins, lacquers, and varnishes in the making of dyes for essential oils in perfumery and for medical substances in pharmacy.
Methanol
Methanol is mixed with ethanol and sold in the market as spirit. Ethanol is the alcohol that is consumed. But to make some of the alcohol unsuitable for drinking, about 5% methanol is added, as methanol is poisonous. This type of spirit is called denatured spirit. Denatured spirit is used in spirit lamps, for disinfectant effects, in wood polish, etc.
Methanol is used as a major solvent for paints and varnishes.Methanol is used for making dyes, perfumes and synthetic fibbers.
Methanol is used for making formaldehyde. Formaldehyde in turn is used for making a type of special insulator called Bakelite.
Methanol is used as a replacement for petrol for environmentally friendly cars and buses.
Ethanol
Ethanol is used for manufacturing of paints, dyes, varnishes.
Ethanol is used in medicines especially for disinfecting area on the skin before giving an injection. It is used fir sterilization of syringes in hospitals.
Ethanol is used for preparation of compounds such as chloroform and ether.
Ethanol is used in thermometers that are used for measuring low temperatures.
Ethanol is used for making spirit levels and in spirit lamps.
Ethanol is used as a substitute for fuels in vehicles that are environmentally friendly. They give off low emissions of carbon monoxide gas that is harmful to the environment.
Ethanol is the main component of alcoholic beverages such as rum, whisky and beer.
Ethanol is used as an organic solvent.
Ethanol is used as in antifreeze a mixture for radiators in cars. Ethanol mixed with water freezes at a lower temperature than water. Such an antifreeze mixture is used for radiators in cold countries.
Home | About | Topics | Resources
Features | Glossary | Quiz